Biden Admin To Roll Out Variant-Specific Boosters — New Vaccines By Fall?
The next generation of COVID-19 booster shots for everyone 12 and up from both Moderna and Pfizer-BioNTech target the strains most responsible for spread in the country.
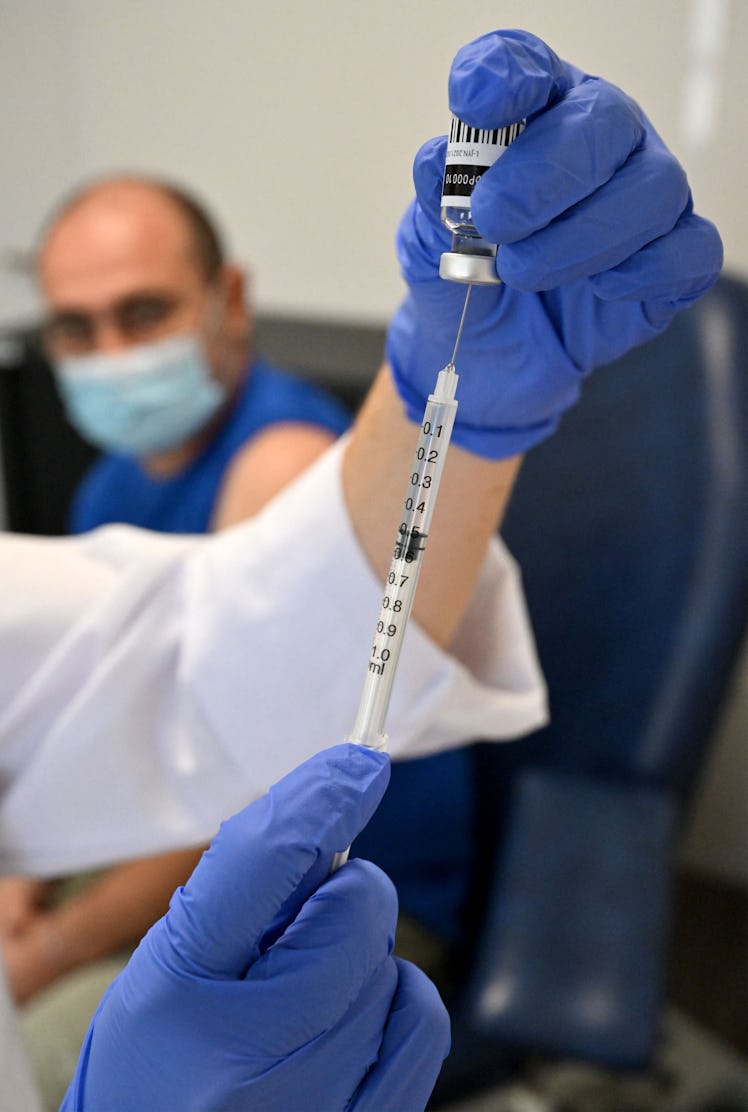
The Biden administration plans to push booster shots for those 12 and older soon after Labor Day, when the COVID-19 virus is likely to circulate more. But these boosters will be different than the ones previously offered. The plan is to begin rolling out updated versions of Moderna's and Pfizer-BioNTech's COVID-19 booster shots that will target specific coronavirus variants.
The Food and Drug Administration (FDA) is planning to authorize the next generation of COVID booster shots. Unlike the original booster, the new booster will target the Omicron BA.4 and BA.5 variants in addition to the original strain of the virus, according to CNN Health. Nearly 89% of all COVID cases in the U.S. are caused by the BA.5 variant, according to the Centers for Disease Control and Prevention (CDC). BA.4 is the cause of many of the remaining cases.
Moderna is seeking authorization for its updated booster for all adults 18 years old and up, while Pfizer is seeking authorization for people 12 years old and up. The Moderna booster will a 50-microgram dose, the same as the company’s original vaccine series, and Pfizer’s will be a 30-microgram dose, the same as the company’s first booster.
According to The New York Times, Dr. Peter Marks, a top vaccine regulator for the FDA, said authorization for the updated booster isn't far away. Authorization which would need to be followed by CDC approval. According to CNN, the CDC's Advisory Committee on Immunization Practices panel plans to meet on Sept. 1 and Sept. 2 to discuss the updated COVID boosters. The agency could sign off on the updated boosters shortly after the meeting.
The applications sent to the FDA include data on how well the boosters performed. Still, they weren't tested in human trials, unlike the original vaccine. "The decision to move forward without complete data from human trials has been a sticking point for some outside scientists," NBC News reports, "who say the new shots have not demonstrated that they are any better than the existing vaccines from Pfizer and Moderna.
However, even though human trials haven't been completed, the FDA is confident in the boosters. Dr. Marks said the agency has "extremely good" data showing the boosters are effective and safe. "How confident am I?" he added. "I'm extremely confident."
Should the approval timeline go according to plan, the updated vaccination boosters would be ready to administer as early as the day after Labor Day, ahead of the expected late fall and winter surge.